Today we have two inspirational news stories! An exciting potential COVID-19 treatment, Remdesivir by Gilead, is showing promise in clinical trails ( full reports included). Emergency access to Remdesivir outside of clinical trials for individual compassionate use is possible. For riders, Polaris is celebrating unsung heroes with a great contest to win one of four Polaris Slingshot three month rentals. Contest entry is easy, just share your stories about a medical professional, a first responder, grocery worker, an educator learning to teach from a far, a seamstress supporting a local medical facility, a young adult delivering food to the community, or a stay-at-home mom turned school teacher!
I hope you enjoy this weeks Inspirational stories on Total Motorcycle. We will continue to do this each and every Friday if you like the idea!
We would like to thank Polaris and Gilead for their positive contributions in these tough times and for inspiring us to create this weeks stories for you. If you would like to learn more about Polaris, Slingshot or Gilead’s COVID-19 treatment read below or click on the links for further information.
POLARIS SLINGSHOT TO CELEBRATE AND RECOGNIZE TODAY’S UNSUNG HEROES WITH A SUPERHERO RIDE
In a time of unprecedented events, millions of Americans are going above and beyond; whether it’s a battle through new adversity in daily life or helping someone in need. In an effort to shine a light and celebrate these individuals, Polaris Slingshot is asking its community to share stories about the superheroes in their lives.
Whether a medical professional, a first responder, grocery worker, an educator learning to teach from a far, a seamstress supporting a local medical facility, a young adult delivering food to the community, or a stay-at-home mom turned school teacher – Slingshot wants to hear these stories and say “thank you.” From now until June 3, Slingshot will be celebrating and acknowledging these local heroes on its social media channels. And to show appreciation, the company is awarding four of the submitted heroes a free three-month rental of an all-new 2020 Slingshot roadster.
“During these unprecedented times, Americans have had to adapt and stretch to unimaginable lengths,” said Chris Sergeant, Vice President of Polaris Slingshot. “Everyday tasks are more challenging. Jobs are significantly more demanding. These are the individuals we want to show our gratitude for, share their story and, in some cases, reward them with a free three-month Slingshot rental. Because every superhero deserves a superhero ride.”
To submit, anyone can post to Facebook, Instagram or Twitter using the hashtag #SlingshotSuperhero. In the post, submitters should explain the hero’s story and how they are battling through adversity, lending support, or committed to their trade during these trying times. Slingshot will highlight a number of submissions throughout the program, while one winning submission will be awarded every two weeks until early June. Consumers can also register online at: https://slingshot.polaris.com/en-us/nominate-a-superhero/. Prize fulfillment will come at a time when the winner can get out and fully enjoy all the fun Slingshot has to offer.
An Open Letter from Gilead Chairman & CEO
Earlier today, the New England Journal of Medicine (NEJM) published an analysis of the effects of our investigational medicine remdesivir on a small group of patients with severe symptoms of COVID-19.
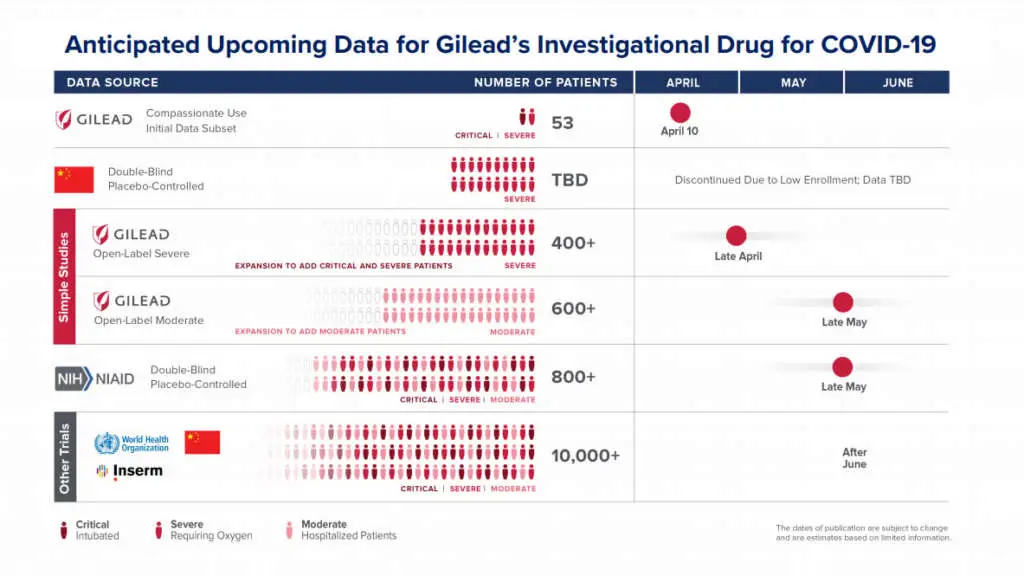
These are patients who received treatment through the compassionate use program for remdesivir, which is for critically ill patients who are unable to take part in a clinical trial. The results, which cover 53 of the first patients to have been treated in the program, show that the majority demonstrated clinical improvement after taking remdesivir. We recognize the limitations of these compassionate use data from a purely investigational perspective, while knowing they are of the greatest significance for the patients whose symptoms improved. These early data from 53 patients have not been generated in a clinical trial and cover only a small portion of the critically ill patients who have been treated with remdesivir.
Remdesivir is an investigational treatment and has not been approved for use anywhere in the world. In the broader efforts to determine whether it is a safe and effective treatment, we have some way to go. Multiple clinical trials are underway across the world to build a complete picture of how remdesivir works in various contexts. These studies cover a range of patient populations across different demographics and with varying types of symptoms: moderate, severe where patients need oxygen support, and critical where medical ventilation is required. These patients all receive remdesivir through intravenous infusions in a hospital setting.
In studying remdesivir, the question is not just whether it is safe and effective against COVID-19 but in which patients it shows activity, how long should they receive treatment and at what stage of their disease would treatment be most beneficial. Many answers are needed, which is why we need multiple types of studies involving many types of patients.
Some of these answers will start to emerge in the coming weeks as we receive the first data from the various clinical trials underway.
Clinical trials for remdesivir
Seven clinical trials have been initiated to determine whether remdesivir is a safe and effective treatment for COVID-19. Each of these was set up with unprecedented speed thanks to the remarkable efforts of the various groups involved, as well as the level of knowledge we had on remdesivir.
To some extent, the trials have had to be adaptive in design as our understanding of the disease itself continues to evolve. The virus emerged and spread at an intense speed and everyone is working quickly to understand it. Our interpretation of the results will also be shaped by what we continue to learn about the disease.
The order in which the trials were initiated mirrors the path of the pandemic. China initiated the first two studies in early February for patients with severe and moderate symptoms of the disease. Since then, an additional five trials have been initiated around the world.
Two Phase 3 studies are being run by Gilead in areas with a high prevalence of COVID-19 in the United States, Asia and Europe. One of these is for patients with severe disease and the other studies remdesivir in patients with more moderate symptoms. One of the many questions that these studies aim to answer is whether treatment duration can be shortened from 10 days to 5 days. The severe arm fully enrolled the number of patients it was originally designed for and we have now expanded the study so that thousands more patients can participate, including those on mechanical ventilation.
The U.S National Institute of Allergy and Infectious Disease (NIAID) began a global trial on February 21. This trial randomly assigns patients to treatment with either remdesivir or with a placebo to enable a controlled comparison of outcomes. The trial is enrolling approximately 800 patients with a broad spectrum of symptoms.
The World Health Organization is also conducting a global trial, Solidarity, and the Inserm DisCoVeRy trial recently began in Europe. A summary of remdesivir trials with upcoming data readouts can be found here.
We know that there is tremendous interest around when the data from these trials will be available and what they will tell us about remdesivir. We feel the urgency as we wait for the science to speak. With every day that goes by, the desperate need to equip healthcare workers and their patients with a safe, effective treatment becomes more pressing. We are working with intense speed to determine whether remdesivir could be an option and we are committed to sharing information when it becomes available to us.
We expect that we will have preliminary data from the study of remdesivir in severe patients at the end of April and will work quickly to interpret and share the findings. The publication of data from the China remdesivir trials rests with the Chinese investigators, but we have been informed that the study in patients with severe symptoms was stopped due to stalled enrollment. We look forward to reviewing the published data when available. In May, we anticipate the initial data from the placebo-controlled NIAID trial as well as data from the Gilead study of patients with moderate symptoms of COVID-19.
To a large extent, the timelines are determined by epidemiology and the numerous challenges that come with studying a treatment for a newly emerged disease. As with so much in this pandemic, this is unchartered territory for many of us involved in the process.
Ongoing collaboration
While it may feel like a long wait for data given the urgency of the situation, it has been only two months since the first clinical trials began. Given that it can take a year or more to have the first clinical data for an investigational treatment, it is remarkable that we expect to have the first remdesivir trial data so soon.
This speed is the result of strong collaboration and immense dedication among the many groups involved, from regulatory authorities to hospital administrators, clinicians and study investigators. As with all the work on remdesivir, everyone is driven by the same sense of urgency and a commitment to maintaining scientific rigor throughout.
All of us at Gilead are grateful to the many groups and organizations who are collaborating to find answers on remdesivir and above all, to the physicians and patients involved in the clinical trials. When we talk about trial results, we tend to think in terms of numbers, trends and statistics. We realize that behind each of these numbers is a patient who has agreed to take part in a trial and share the data from their personal experience. It is thanks to the thousands of patients like these and the physicians who are treating them, that we will be able to determine whether remdesivir can be used safely and effectively for many more patients in the future.
Emergency Access to Remdesivir Outside of Clinical Trials
Remdesivir is an investigational drug that has not been approved by any regulatory authority, and the safety and efficacy of remdesivir for the treatment of COVID-19 are not yet known. Enrollment in clinical trials is the primary way to access remdesivir to generate critical data that inform the appropriate use of remdesivir. We recognize that enrollment in clinical trials is not feasible for all patients, and in consultation with regulatory authorities we have implemented programs that are designed to provide emergency treatment access for patients with severe clinical manifestations of COVID-19.
Gilead is currently in the process of transitioning the provision of emergency access to remdesivir from individual compassionate use requests to expanded access programs in countries around the world in order to mange the volume of requests, expedite the delivery of the drug for patients, accelerate the gathering of data outside of clinical trials and, ultimately, should the data be positive, be in a position to more quickly apply for potential regulatory approvals that can enable broader access to remdesivir.
An expanded access program is now in place in the United States. A list of active sites in the United States is available and regularly updated here.
Gilead will continue to work with regulatory authorities worldwide to introduce expanded access programs. The European Medicines Agency has adopted Article 83 providing recommendations for the use of remdesivir for emergency treatment via expanded access programs in the European Union. We will be opening expanded access programs (cohort compassionate use) in the United Kingdom, France, Germany, Spain, Italy and Switzerland, subject to national regulatory approvals.
Individual compassionate use requests continue to be reviewed for pregnant women and children less than 18 years of age with confirmed COVID-19 and severe manifestations of disease.
Remdesivir Clinical Trials
Gilead-initiated Trials
Gilead has initiated two Phase 3 clinical studies to evaluate the safety and efficacy of remdesivir in adults diagnosed with COVID-19 following the U.S. Food and Drug Administration’s (FDA) rapid review and acceptance of Gilead’s investigational new drug (IND) filing. These randomized, open-label, multicenter studies began enrolling patients in March 2020 and will enroll a total of approximately 1,000 patients in the initial phase of the studies, in countries with high prevalence of COVID-19.
The first of two studies will evaluate the safety and efficacy of both a 5-day and a 10-day dosing duration of remdesivir, in addition to standard of care, for patients with severe manifestations of COVID-19. The second study will evaluate the safety and efficacy of the same dosing regimens of remdesivir in addition to standard of care for patients with moderate manifestations of COVID-19, compared with standard of care alone.
Other Trials
Health authorities in China have initiated two clinical trials in patients who have been infected with COVID-19 to determine the safety and efficacy of remdesivir as a potential treatment for the coronavirus. The two studies are being coordinated by the China-Japan Friendship Hospital and are being conducted at multiple sites in Hubei province. Gilead is providing study drug at no charge and provided input on study design and conduct.
One study is evaluating remdesivir in patients with confirmed disease who have developed more severe clinical manifestations such as a requirement for supplemental oxygen. The other study is evaluating remdesivir in patients with confirmed COVID-19 infection who have been hospitalized but are not displaying significant clinical manifestations of disease such as an oxygen requirement.
The U.S. National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, has initiated a Phase 2 adaptive, randomized, double-blind, placebo-controlled trial into remdesivir as a potential treatment for hospitalized adult patients diagnosed with COVID-19. Gilead is providing study drug at no charge and provided input on study design and conduct.
Development of Remdesivir
Remdesivir was invented by Gilead building on more than a decade of our research. Over that time, our research scientists have explored the compound for multiple potential uses to help address urgent and unmet medical needs around the world, including Ebola, SARS, Marburg, MERS and most recently COVID-19. Our antiviral expertise is the result of more than 30 years of research and the investment of billions of dollars in research and development.
Gilead’s antiviral work reflects its commitment to collaborating with the global health community and advancing potential treatments that may help in the global response to public health emergencies.
In January 2020, when a new pneumonia-like illness in China was identified as a coronavirus, Gilead moved quickly to determine whether remdesivir could play a role in responding to the growing public health threat that subsequently became known as COVID-19. Gilead’s preclinical data suggested that testing remdesivir against COVID-19 should take place immediately.
• Gilead’s team of virologists quickly generated the preclinical data to characterize remdesivir’s activity against the new COVID-19 virus and to determine the potential benefit of further testing.
• In January 2020, Gilead provided remdesivir to the China CDC to test the compound against isolates of the virus that causes COVID-19 through their independent antiviral assays. Gilead provided remdesivir to U.S. academic institutions in February 2020 for similar testing. Results are expected soon.
• In February 2020, Gilead began supporting multiple clinical trials to evaluate the safety and efficacy of remdesivir as a potential treatment for COVID-19.
• Gilead donated study drug and provided scientific input for two clinical trials coordinated by the China-Japan Friendship Hospital in China, which began enrolling patients in early to mid- February.
• Gilead donated drugs and provided scientific input for a NIAID-initiated global clinical trial of remdesivir in late February, including the first site to enroll patients in the United States.
• In late February, Gilead initiated its own two Phase 3 studies of remdesivir, which will enroll patients in countries globally with high numbers of diagnosed COVID-19 cases. These studies began enrolling patients in March 2020 and will evaluate two dosing durations of remdesivir.
• Gilead has provided input on the design of both WHO’s global Solidarity trial and the INSERM-sponsored DisCoVeRy trial in Europe. These trials have already initiated and will expand to additional countries over the coming months. Gilead has committed to providing remdesivir to support these studies, which will be conducted in more than 70 countries worldwide.
• In anticipation of potential future needs, we have accelerated manufacturing timelines to increase our available supply as rapidly as possible. We are doing this before knowing whether remdesivir will be determined to be safe and effective to treat patients with COVID-19.